Linaclotide Acetate
Appearance: White or almost white crystalline powder
Standard: in-house
Application: Cosmetic Grade
Supply Ability:50kg per month
Sequence:H-Cys-Cys-Glu-Tyr-Cys-Cys-Asp-Pro-Ala-Cys-Thr-Gly-Cys-Tyr-OH, cyclic (1-6), (2-10), (5-13)-tris(disulfide)
Purity: ≥98%
Payment: T/T, LC or DA
Delivery Time: Ready Stock in Local Warehouse, 1-3 days
Origin: China
Shipping: DHL, FedEx, TNT, EMS, By Sea, By Air
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
We supply Linaclotide Acetate
Linaclotide Acetate is a high- quality product that we offer. It's a potent and picky agonist of guanylate cyclase- C, which plays an important part in regulating intestinal fluid stashing and motility. Linaclotide has been shown to be effective in the treatment of perverse bowel pattern with constipation( IBS- C) and habitual idiopathic constipation( CIC). This product is manufactured using state- of- the- art technology and is guaranteed to meet the loftiest quality norms. communicate us moment to learn further about Linaclotide and how it can profit your business.
What's Linaclotide Acetate?
Linaclotide Acetate is a white to gray white unformed greasepaint; Slightly answerable in water and sodium chloride waterless result(0.9). Acetyllinalopide is a synthetic peptide structure containing 14 amino acids, associated with the endogenous guanosine peptide family. It's the only clinically approved GC- C( guanylate cyclase- C) agonist medicine for original intestinal action, and can be used to treat adult cases with constipated perverse bowel pattern( IBS- C) and habitual idiopathic constipation( CIC).
Basic Information
Product Name: Linaclotide
Sequence: H-Cys Cys Glu Tyr Cys Asp Pro Ala Cys Chr Gly Cys Tyr OH, cyclic (1-6), (2-10), (5-13) tris (disulfide)
CAS: 851199-59-2
MF:C59H79N15O21S6
MW:1526.74
EINECS:251-228-4
MDL No.:MFCD20526656
Structural formula:

Origin: China
Application field: Used for treating adult chronic idiopathic constipation and constipated irritable bowel syndrome (IBS-C)
Delivery Time: in stock
Technical Specification
|
ITEM |
SPECFICATION |
RESULTS |
|
Appearance |
White or almost white crystalline powder |
Complies |
|
Identity by HPLC |
The reaction is same with the reference |
Complies |
|
Amino Acid Composition |
±20%of theoretical |
Complies |
|
Purity (HPLC) |
≥98.0% |
99.5% |
|
Related Substance |
Total Impurities(%)≤2.0% |
0.5% |
|
Largest Single Impurity(%)≤1.0% |
1.0% |
|
|
Acetate Content(By HPLC) |
≤15% |
Complies |
|
Water Content(By HPLC) |
≤5.0% |
3.0% |
|
Bacterial Endotoxins |
≤5IU/mg |
Complies |
|
Conclusion: |
Conform with enterprise specification. |
|
Package: 1g; 5g;100g or according to your demands
Storage: Preserve in tight,light-resistant containers in a cool place
Shelf Life: 24 months
Mechanism of action
Linaclotide Acetate is a guanylate cyclase- C receptor agonist( GCCA) with both visceral analgesic goods and endocrine exertion. Linalotide and its active metabolites can bind to the guanylate cyclase- C( GC- C) receptor on the face of the small intestinal epithelial lumen. In beast models, through the activation of GC- C, linalopide can palliate visceral pain and ameliorate gastrointestinal transport speed. In humans, this medicine can also increase colon transport speed. The result of GC- C activation is an increase in intracellular and extracellular cGMP( cyclic guanosine) attention. Extracellular cGMP can reduce the exertion of pain whim-whams filaments and palliate visceral pain in model creatures. Intracellular cGMP can increase the stashing of chloride and bicarbonate in the small intestine by cranking CFTR( cystic fibrosis transmembrane conduction controller), eventually leading to increased stashing of small intestine fluid and briskly intestinal transport speed.
Pharmacodynamics
Although the pharmacological goods of LINZESS haven't been completely estimated in humans in clinical studies, LINZESS has shown harmonious changes in coprolite conformation score( BSFS) using Bristol and increased coprolite frequence dimension.
Pharmacokinetics
immersion After oral administration, LINZESS has low immersion and low bioavailability. The attention of linalopide and its active metabolites in tube after oral administration of 145 μ g or 290 μ g is below the quantification limit. thus, standard pharmacokinetic parameters similar as area under wind( AUC), outside attention( Cmax), and half- life( t?) can not be calculated.
Distribution Given that the blood attention of oral boluses of linalopide for treatment can not be measured, it's anticipated that linalopide will be distributed to veritably little towel.
Metabolism Linalotide is metabolized as its main active metabolite in the gastrointestinal tract by losing its terminal tyrosine half. Linalotide and its metabolites are proteolytic and degraded into lower peptides and naturally being amino acids in the small intestine.
Elimination After feeding and dieting, subjects were given 290 μ g of LINZESS daily for a aggregate of 7 days. The average recovery of active peptides in fecal samples was about 5%( fasting) and about 3%( eating), and in fact, all were active metabolites.
Food impact In a crossover study, 18 healthy subjects were given LINZESS290 μ g in both eating and non eating countries for a aggregate of 7 days. No active metabolites of linalopide were detected in the tube. Taking LINZESS incontinently after a high- fat breakfast results in advanced frequence of loose droppings and bowel movements compared to taking it on an empty stomach( see lozenge and administration styles(2.1,2.2)). In clinical trials, LINZESS is administered on an empty stomach at least 30 twinkles before breakfast.
Preparation method
Semi selective oxidation to form three pairs of disulfide bonds for the synthesis of linalopide. Using the Fmoc solid-phase synthesis strategy, Wang resin was used as a carrier to synthesize three linear precursor compounds of [4Trt+2Acm] and three [2Trt+4Acm] linalopide. While cracking the resin, the Trt protective group on cysteine was removed, and partial disulfide bonds were formed using the catalytic oxidation method of hemin chloride. In the next reaction, a diphenylsulfoxide [PhS (O) Ph]/trimethylchlorosilane (CH3SiCl3) system was used to remove the Acm protective group in TFA and form the remaining disulfide bonds. The synthesized 6 Among the linear precursor peptides, three were able to obtain linalopide with high conversion rates, and Acm protected cysteine was successfully applied to the synthesis of linalopide.
(1) The coupling of the first amino acid and resin in the synthesis of peptides: Weigh Wang resin (1g, 0.72mmol/g) in a 25ml solid-phase reactor and swell with 15ml CH2Cl2 for 2 hours. Weigh Fmoc Tyr (tBu) (0.99g, 2.16mmol), HBTU (0.82g, 2.16mmol), and HOBT (0.29g, 2.16mmol) and dissolve them in 15ml of DMF. After activation with DIEA (1.82ml, 11.04mmol), add them to a solid-phase reactor and shake at room temperature for 3 hours. Add acetic anhydride: pyridine: DMF=2:1:3 (V/V/V) (1 × 30min) for sealing, and wash the resin sequentially with DMF (4 × 1min), CH2Cl2 (4 × 1min), and DMF (4 × 1min). Coupling of remaining amino acids: Following the amino acid sequence of linalopide, repeat the above steps for coupling and Fmoc removal. After each amino acid is conjugated, use Kasier reagent for detection, and detect proline (Pro) using bromophenol blue method. If the color reaction is negative, proceed to the next coupling cycle. Otherwise, repeat the coupling step. Peptide lysis: After coupling is completed, weigh the peptide resin and put it into a reaction flask. Prepare the lysis solution in the ratio of 1g resin and 5ml lysis agent. Under nitrogen protection, add TFA: water: benzyl sulfide: phenol: EDT=82.5:5:5:2.5 (V/V/V/V/V). After 2 hours of lysis, filter and wash with a small amount of TFA and CH2Cl2 three times. Combine the filtrate. Blow off 3/4 of the volume of the obtained filtrate with nitrogen and drop it into a large amount of ice ether to precipitate white flocculent precipitate. Centrifuge to obtain the corresponding crude peptide.
(2) Purification and analysis of peptides: The crude peptides obtained will be dissolved in a small amount of water and purified using Waters600 semi preparative liquid phase and XB-C18 preparation column. Under high-performance liquid phase conditions, the mobile phase A is water (containing 0.1% volume fraction of TFA); Mobile phase B: acetonitrile (containing 0.1% volume fraction of TFA); Mobile phase gradient: Mobile phase B15% to 50% (volume fraction), 20 minutes; The flow rate is 3mL/min; Detect at a wavelength of 220nm. Collect the main peak, rotate and evaporate to remove acetonitrile, and freeze dry to obtain pure peptide.
(3) The formation of disulfide bonds by catalytic oxidation of heme chloride to form disulfide bonds: Weigh the linear precursor peptide of linalopide (3mg, 2 μ mol) with Trt protective group removed, dissolve it in a buffer solution of Tris HCl at pH 8.0 (5mL), add 20% (by volume) of heme chloride (containing a small amount of DIEA), stir at room temperature and open for 2 hours, and analyze the reaction by HPLC at the end of the reaction PhS (O) Ph/CH3SiCl3 removes Acm protective groups and forms disulfide bonds: Weigh the peptide (0.6mg, 0.4 μ mol), dissolve it in TFA (0.4mL), add CH3SiCl3 (12 μ L, 250 equiv.), PhS (O) Ph (0.8mg, 10 equiv.), benzyl ether (4.3 μ L, 100 equiv.), stir at room temperature and open for 20 minutes, add dry ether (10mL), shake with 4 mol/L acetic acid (5mL), take the aqueous phase for HPLC analysis and separation.

The figure shows the route for the formation of linalopide containing three pairs of disulfide bonds through semi selective oxidation strategy
Application
Used for the treatment of irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC).
Payment Term

Why Choose Xi'an Yihui?
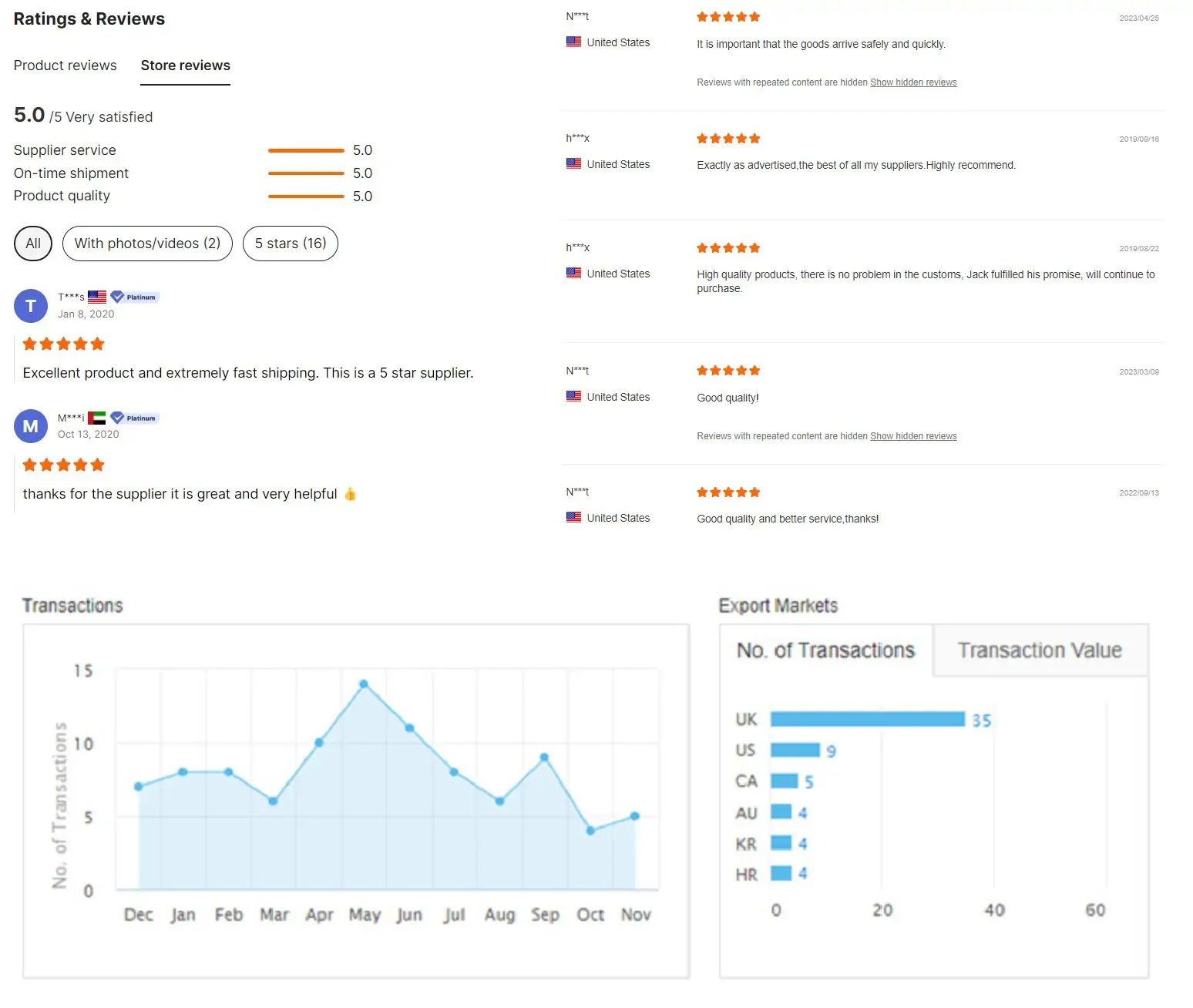
Customer feedback
Xi'an Yihui certificates

Our advantage
Rich experience: we have 13 Years of professional experience;
Customers all over the world: sell to more than 100 countries;
Provide diversified products: the products have been applied to all major international brands in the fields of drugs, dietary supplements, cosmetics, animal nutrition, and functional food.
Price advance: low MOQ with competitive price;
Quality certification: ISO; Halal; Kosher certified
After-sales service: Professional team 7*24 hours customer service.
Where to buy?
In summary, as a professional Linaclotide Acetate manufacturer, we have advanced technology, scale advantage, the best quality, rich production experience, and excellent services. These advantages will make it stand out in the market competition and win more customer trust and support.
If you want to know more about it or are interested in purchasing it, you can contact us at any time. We will reply to you as soon as possible after we see the message.
our contact information:
E-mail: sales@yihuipharm.com
Tel: 0086-29-89695240
WeChat or WhatsApp: 0086-17792415937
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.











