Olopatadine hydrochloride API
Appearance: White crystalline powder
Standard: in-house
Application: Anti allergy Supply Ability: 500kg per month
Purity: ≥99%
Payment: T/T, LC or DA
Delivery Time: Ready Stock in Local Warehouse, 1-3 days
Origin: China
Shipping: DHL, FedEx, TNT, EMS, By Sea, By Air
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
We supply Olopatadine hydrochloride API
Our company supplies high- quality Olopatadine hydrochloride API. it is an antihistamine drug used to treat antipathetic conditions similar as hay fever, hives, and eye disinclinations. It works by blocking the action of histamine, which is a natural substance produced by the body that causes antipathetic symptoms. Our API is manufactured using state- of- the- art processes and is biddable with assiduity norms. We offer competitive pricing and fast delivery times, making us the ideal choice for those in need of dependable it. communicate us moment to learn further.
What's Olopatadine hydrochloride?
Olopatadine Hcl API is a white or gray white crystalline greasepaint with the molecular formula C21H23NO3 and a molecular weight of 337.41200. It has a wide range of pharmacological goods, similar as histamine H1 receptor antagonist, chemical middleman inhibition, tachykinin release inhibition, and eosinophil infiltration inhibition. Suitable for itching caused by antipathetic rhinitis, urticaria, and skin conditions.
Olopatadine hydrochloride is an anti antipathetic medicine developed and retailed by Japanese turmoil company Concord, and has been listed in multiple countries including Europe, the United States, and Japan. It has a binary effect of inhibiting histamine release and widely envenoming HI receptors, without the common central nervous system inhibition and cardiac poisonous side goods of HI receptor antagonists. Oral use of Chemicalbook is used for the treatment of antipathetic rhinitis, skin vexation, polymorphic exudative erythema, and urticaria. Its eye drops are used to treat antipathetic conjunctivitis. This medicine can inhibit the release of histamine from mast cells and widely envenom H1 receptors. It has no effect on nascence adrenergic receptors, dopamine receptors, and M1 and M2 receptors, but has smaller side goods on the central nervous system. It's a new generation of preferred anti antipathetic medicines.
Basic Information
Product Name: Olopatadine hydrochloride
CAS: 140462-76-6
MF:C21H24ClNO3
MW:373.88
EINECS:604-185-4
MDL No.:MFCD00875716
Structural formula:

Application: Anti allergy
Origin: China
Delivery Time: in stock
Technical Specification
|
Sr. No. |
Items |
Specifications |
Results |
|
01. |
Appearance |
White crystalline powder |
White crystalline powder |
|
02. |
Solubility |
This product is dissolved in methanol, soluble in water, and soluble in ethanol. |
Conforms |
|
03. |
Identification |
||
|
a. By UV |
according with P23-Z CRS. |
Conforms |
|
|
b. By HPLC |
the retention time of the major peak according with P23-Z CRS. |
Conforms |
|
|
04. |
Related substances (% Area Normalization By HPLC) |
||
|
a. Any single impurity |
NMT: 0.50% |
0.00% |
|
|
b. Total impurities |
NMT: 1.00% |
0.00% |
|
|
05. |
Residual solvents |
||
|
a. Methanol |
NMT: 3000 ppm |
N D |
|
|
b. acetone |
NMT: 5000 ppm |
1739 ppm |
|
|
c. tetrahydrofuran |
NMT: 720 ppm |
N D |
|
|
d. Toluene |
NMT: 890 ppm |
N D |
|
|
e. DMF |
NMT: 1090 ppm |
N D |
|
|
06. |
Heavy metals |
NMT: 20ppm |
Conforms |
|
07. |
Residue on ignition |
NMT: 0.10% |
0.05% |
|
08. |
Clarity and color of solution |
according with P23-Z CRS. |
Conforms |
|
09. |
Loss on drying (at 105℃, till constant weight) |
NMT: 0.50% |
0.07% |
|
10. |
Assay (on dried basis, by titration) |
NLT: 98.5% |
99.8% |
|
The product CONFORMS to the above specifications. |
|||
Package 100g; 1kg, or according to your demands
Storage conditions: In an airtight container Protected from light
Transportation conditions: Transportation at room temperature
Shelf Life: 24 months
Pharmacological action
Olopatadine hydrochloride API belongs to the derivations of dibenzooxate, and the introductory groups in the patch have a strong negative effect on receptors. It also inhibits the release of seditious intercessors by mast cells, prostrating central dreamy side goods by introducing hydrophilic carboxylic acid groups.
Indication
Oral treatment for antipathetic rhinitis, rubella, itchy skin conditions similar as eczema, dermatitis, prurigo, skin itching, psoriasis vulgaris, colorful forms of exudative erythema, skin vexation, polymorphic exudative erythema, and measles. 1 eye drops for the treatment of antipathetic conjunctivitis.
Application
Olopatadine Hcl API is a binary amusement histamine H1 receptor antagonist and mast cell stabilizer. Anti antipathetic medicines; Antihistamines.
Preparation process
1) The medication of 4-( 2- carboxybenzyloxy) phenylacetic acid( 4) was carried out in a 250mL three necked beaker.10.0 g(0.06 spook) of p- hydroxyphenylacetic acid,8.1 g(0.06 spook) of phthalein, and 60mL of DMF were added, stirred and dissolved.7.0 g(0.13 spook) of potassium hydroxide methanol result was added dropwise, and the dropwise addition was completed. The influx response was carried out for 1 hour, and the methanol was faded. also, the temperature was raised and the influx response was carried out for 3 hours. The response result was cooled and poured into 50mL of water. A large quantum of white precipitate was generated by conforming the pH to 4mol/ L hydrochloric acid result. The precipitate was also left to stand for 1 hour, filtered to gain a white solid, washed with water, dried, and recrystallized with ethanol to gain a white solid.
2) The medication of 6,11- dihydrobenzo( b, e) oxo-11-keto-2-ethyl acetate( 5) was carried out in a 100mL three necked beaker. 25g of phosphorus pentoxide was added, and22.7 mL of absolute ethanol was sluggishly dropped at room temperature. The response was also hotted
to 110 ℃ for 1 hour.10.0 g( 33mmol) of 4-( 2- carboxybenzyloxy) phenylacetic acid( 4) was added, and the response was carried out at 110 ℃ for 2 hours. The admixture was cooled and poured into ice water for birth with ethyl acetate( 30mL × 3). The ethyl acetate layers were combined, washed with water to pH 7, dried with anhydrous sodium sulfate, and the detergent faded to gain9.4 g of brown unctuous substance with a yield of 90%.
3) Preparation of 6,11- Dihydrobenzo( b, e) oxa-11-keto-2-acetic acid( 6) Place the brown unctuous substance attained in the former step in a 100mL eggplant shaped beaker, add 20mL of 4mol/ L sodium hydroxide result, influx response for 1 hour, cool, acclimate pH 5 with 2mol/ L hydrochloric acid result, excerpt with ethyl acetate( 20mL × 3), combine with ethyl acetate, marshland with water to pH 7, dry with anhydrous sodium sulfate, dematerialize the detergent, and gain a unheroic thick substance. Recrystallize with ethyl acetate and ether, decolorize with actuated carbon, and gain7.1 g of light unheroic chargers with a yield of 83%.
4) Preparation of cis)-11-( 3- dimethylaminopropyl) -6,11-dihydrobenzo( b, e) oxo-2-acetic acid( 7) in a 250mL three necked beaker.14.4 g( 28mmol) of 3- dimethylaminopropyl triphenylphosphohydrobromate and 80mL of THF were added. Under cooling in an ice swab bath, 40mL of1.92 spook/ L n- hexane result of n- butyl lithium was sluggishly added. After adding, the response was stirred below 0 ℃ for 1 hour. 4g( 14mmol) of emulsion 6 was added to a 20mL THF result, which was hotted and refluxed for 12 hours. The admixture was cooled and the THF was faded. The residue was dissolved in 20mL of water. prize with ether( 15mL × 2), adulterate the waterless result with concentrated hydrochloric acid to pH 2, excerpt with ethyl acetate( 15mL × 2), neutralize the waterless result with 4mol/ L sodium hydroxide result to pH 7, concentrate to remove water, and gain3.1 g of crude emulsion 7 with a yield of 65%. The crude product was recrystallized doubly with isopropanol, yielding( Z)-70.97 g with a yield of31.2%.
5) Preparation of Olopatadine hydrochloride( 1) Dissolve0.45 g of the set Olopatadine in 2mL of isopropanol, pass through hydrogen chloride gas, stir at room temperature for 1 hour, concentrate, and recrystallize the residue with acetone to gain0.25 g of white fine solid with a yield of 50%, at 251 ℃( corruption).
Packing & Shipping
|
Packing: 1kg/foil bag;5kg/carton;25kg/fiber drum; or packing as your request. Customization: l Customized logo l Customized packaging l Graphic customization
Shipping: By Courier; By Air or By Sea, according to your demands |
|
Payment Term

Why Choose Xi'an Yihui?
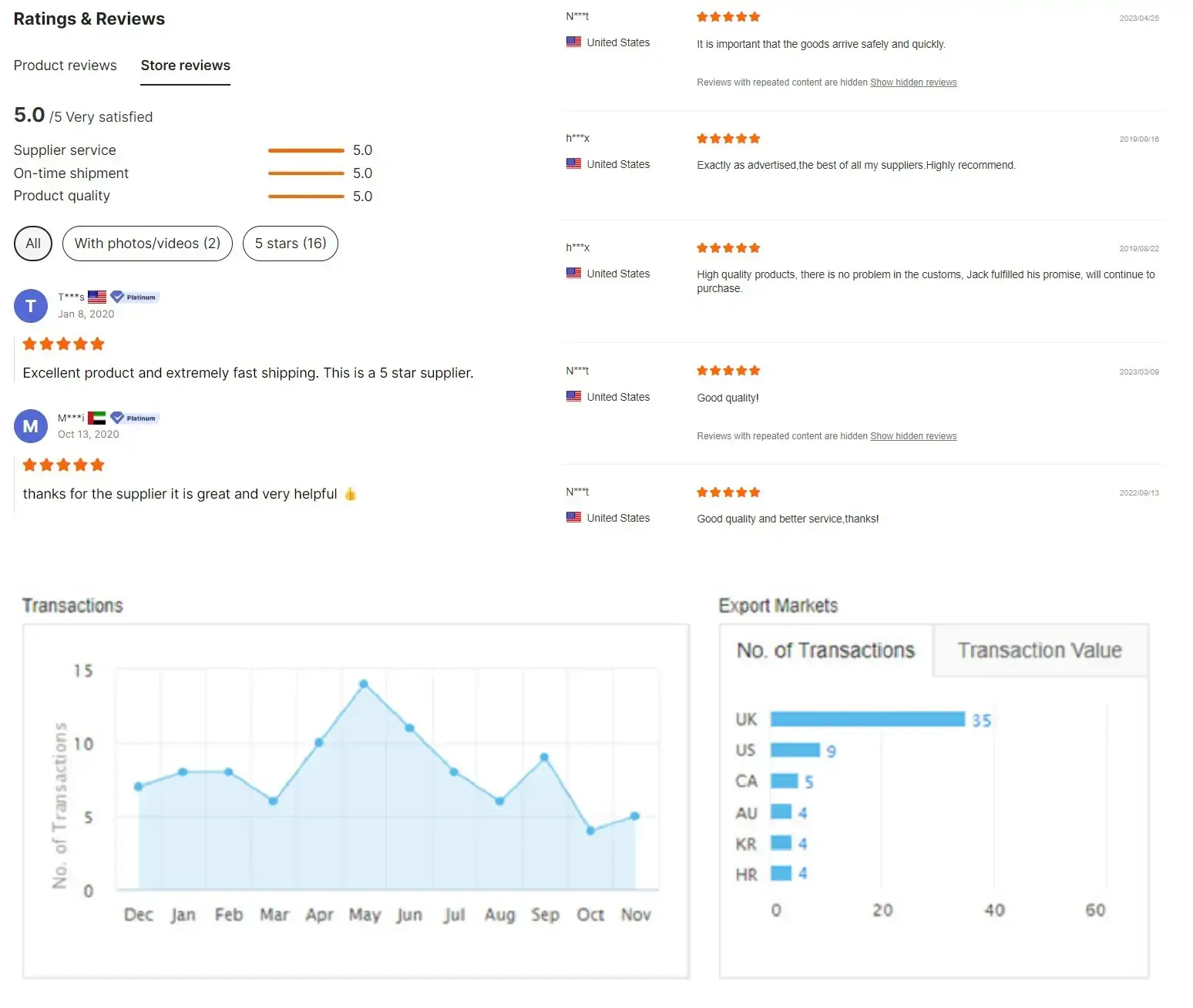
Customer feedback
Xi'an Yihui certificates

Welcome to Xi'an Yihui Factory

Contact Us:
Yihui takes pride in being a trusted Olopatadine hydrochloride API manufacturer and supplier. To acquire this groundbreaking product or discuss your specific requirements, contact us immediately at sales@yihuipharm.com. Rest assured, with Yihui, you're accessing a product of unparalleled quality, backed by certifications that underscore our commitment to excellence in the global market.
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.