Baricitinib API
Appearance: White or off-white powder
Standard: Enterprise standard, 99%
Supply Ability: 100kg per month
Shelf Life: Two years
Payment: T/T, LC or DA
Delivery Time: Ready Stock in Local Warehouse, 1-3 days
Origin: China
Shipping: DHL, FedEx, TNT, EMS, By Sea, By Air
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
We supply Baricitinib API
We're pleased to offer Baricitinib API, a largely effective drug used to treat rheumatoid arthritis and other autoimmune diseases. Our API is sourced from estimable manufacturers, icing the loftiest situations of chastity and quality. it works by blocking enzymes that beget inflammation in the body, furnishing relief for cases with habitual conditions. With our dependable force and competitive pricing, we're confident that our Baricitinib will meet your requirements. communicate us moment to find out further about our product and how we can help you.
What's Baricitinib?
Baricitinib is a white or liquid greasepaint, odorless and tasteless, slightly answerable in water, answerable in hot water, undoable in ethanol or acetone, and fluently answerable in sodium hydroxide test result.
it is an oral asset of JAK kinases 1 and 2, generally used in the treatment of moderate to severe active rheumatoid arthritis( RA) in grown-ups. For rheumatoid arthritis cases who haven't preliminarily used natural DMARDS medicines, Barrectinib can reduce complaint exertion.
A detail History of Research
In February 2017, Barrectinib was approved by the European Union as a monotherapy or in combination with methotrexate for the treatment of moderate to severe active rheumatoid arthritis in adult cases with inadequate or intolerant relief from one or further anti rheumatic medicines( DMARDs). This is also the first JAK asset approved by the European Union for the treatment of rheumatoid arthritis.
In July 2017, the Japanese Ministry of Health, Labour and Welfare approved the use of Barrectinib for the treatment of rheumatoid arthritis cases with poor response to being standard curatives, including forestallment of common structural damage. Kuwait and Switzerland also approved the table of Barectinib in June 2017.
In June 2018, the US FDA approved Barrectinib for the treatment of adult cases with moderate to severe active rheumatoid arthritis who have inadequate response to one or further excrescence necrosis factor( TNF) asset curatives
Basic Information
Product Name: Baricitinib
CAS:1187594-09-7
MF:C16H17N7O2S
MW:371.42
EINECS:691-421-4
MDL No.:MFCD21608464
Structural formula:

Purity: 99%+
Origin: China
Application: Rheumatoid arthritis
Delivery Time: in stock
Technical Specification
|
ITEM OF ANALYSIS |
STANDARD |
RESULT OF ANALYSIS |
|
|
Description |
White or off-white powder |
White powder |
|
|
Identification |
Consistent with the reference spectrum |
Conforms |
|
|
The retention time of the main peak of the test substance should be consistent with that of the reference substance |
Conforms |
||
|
Water |
≤0.5% |
0.12% |
|
|
Residue on Ignition |
≤0.2% |
0.05% |
|
|
Heavy metals |
≤20ppm |
Conforms |
|
|
Related substances |
Single impurity |
≤0.1% |
0.08% |
|
Total impurities |
≤1.0% |
0.19% |
|
|
Solvent residues |
Acetonitrile ≤410ppm |
240ppm |
|
|
Assay |
98.0%~102.0% |
100.1% |
|
|
Conclusion |
Conforms to Enterprise Standard |
||
Package: 1kg; 25kg
Storage conditions: keep tightly closed, store in a cool dry place.
Mechanism of action
Baricitinib API is a selective inhibitor of JAK2 and JAK1, with limited efficacy against JanusKinase, jak3, or TYK2. This kinase and its associated signal transduction and transcriptional activating factors (JAK-STAT) are the main intracellular pathways that control the amplitude and duration of cytokine and type I and II cytokine receptor signaling. These receptors lack intrinsic enzyme activity signal transduction that can be mediated; Janus kinase \ Signal transduction and transcriptional activating factors are activated by JAK enzyme phosphorylation, leading to STAT activation. Several inflammatory factors are involved in the pathogenesis of RA, including IL-6, granulocyte macrophage colony-stimulating factor, and (GM-CSF) factor. The interferon signal is transmitted through the JAK-STAT signaling pathway. Therefore, JAK1 and JAK2 inhibitors can target multiple RA related cytokine pathways, thereby reducing the activation and proliferation of key immune cells in inflammatory cells. It has no effect on JAK3. JAK3 may be different from regular γ Related to chain receptors. Common γ The cytokines in the chain include IL-15 and IL-21, which mainly regulate lymphocyte activation, function, and proliferation.
Application
1. Used for the treatment of moderate to severe active rheumatoid arthritis (RA) in adults. Patients who are intolerant to one or more anti rheumatic drugs can use this product for treatment. This product can be treated as a single drug or in combination with methotrexate. The therapeutic effect on psoriasis, diabetes nephropathy, atopic dermatitis, systemic lupus erythematosus and other diseases is currently in phase II clinical stage.
2. Used in scientific research and chemical reagent fields
Biological Activity
Baricitinib (LY3009104, INCB028050) is a selective inhibitor of JAK1 and JAK2, with IC50 values of 5.9nM and 5.7nM, respectively, which is about 70 and 10 times more selective than JAK3 and Tyk2. It has no inhibitory effect on c-Met and Chk2. Phase 3.
In vitro research
In peripheral blood mononuclear cells, It inhibits the phosphorylation of the typical substrate STAT3 (pSTAT3) stimulated by IL-6 and the subsequent production of chemokine MCP-1, with IC50 values of 44 nM and 40 nM, respectively. It can also inhibit IL-23 stimulated phosphorylation of STAT3 in independent immature T cells, with an IC50 of 20nM.
In vivo research
Baricitinib inhibits IL-6 stimulated STAT3 phosphorylation in whole blood, with an IC50 of 128 nM. it is expected to inhibit JAK1/2 signaling in rats (inhibition rate ≥ 50%) for up to 8 hours. it can inhibit disease scores in adjuvant induced arthritis rat models and has dose-dependent characteristics. Compared with the control group, treatment with it for two weeks can inhibit the volume growth of female deer paws by 50%, and doses of 3mg/kg or 10mg/kg can inhibit more than 95%. Compared with the control group, it treatment can inhibit immune infiltration, edema, and the overall score of periarticular tissue appearance in adjuvant arthritis rat models by 27%, 3mg/kg can inhibit by 64%, and 10mg/kg can inhibit by 82%. it reduced bone resorption by 15%, 61%, and 67% in adjuvant induced arthritis rat models, respectively. it can improve the normal structure and appearance of the ankle and tarsal bones in an adjuvant arthritis rat model through imaging. it can reduce the phosphorylation level of STAT3 in the peripheral blood of Dalayan animals and has dose and time dependent characteristics. it can increase the joint injury comprehensive score of collagen induced arthritis (CIA) model in mice by 47%. In the collagen antibody induced arthritis (CAIA) mouse model, it can reduce pannus (74%) and bone damage (78%), improve cartilage damage (43%) and inflammatory signaling (33%), and increase the overall disease score by 53%. In the CIA and CAIA models, it can inhibit delayed hypersensitivity by 48%. it is effective for patients with active rheumatoid arthritis, while disease modifying drugs and biologics are mostly difficult to be effective. it preferentially inhibits JAK1 and JAK2, with selectivity 210 times higher than Tyk and 3100 times higher than JAK2. The observed effect of GLPG-0634 on ACR20 is at least the same as tofacitinib and superior to it, as it can only moderately affect ACR20 values in one phase II clinical trial. it has dose limited side effects that can induce anemia, which is due to its impact on JAK2, but its efficacy is very clear no matter what.
Reference:
Baricitinib CAS #: 1187594. ChemicalBook [Cited on March 12, 2024]
Baricitinib significantly improves symptoms of moderate to severe rheumatoid arthritis. China National Knowledge Infrastructure [citation date: March 12, 2024]
Ren Guobin, Yi Dongxu, Chen Jinyao Barecytinib A crystal form and its preparation method
Huang Shijie Baricitinib significantly improves symptoms of moderate to severe rheumatoid arthritis. International Journal of Pharmaceutical Research, 2016
Ren Guobin, Yi Dongxu, Chen Jinyao Barrectinib trifluoroacetate B crystal form and its preparation method. 2019
Han Zhidong, Shen Lixian, Cui Bo, Wang Qi, etc A drug composition containing Barecytinib and its preparation method and use
Payment Term

Why Choose Xi'an Yihui?
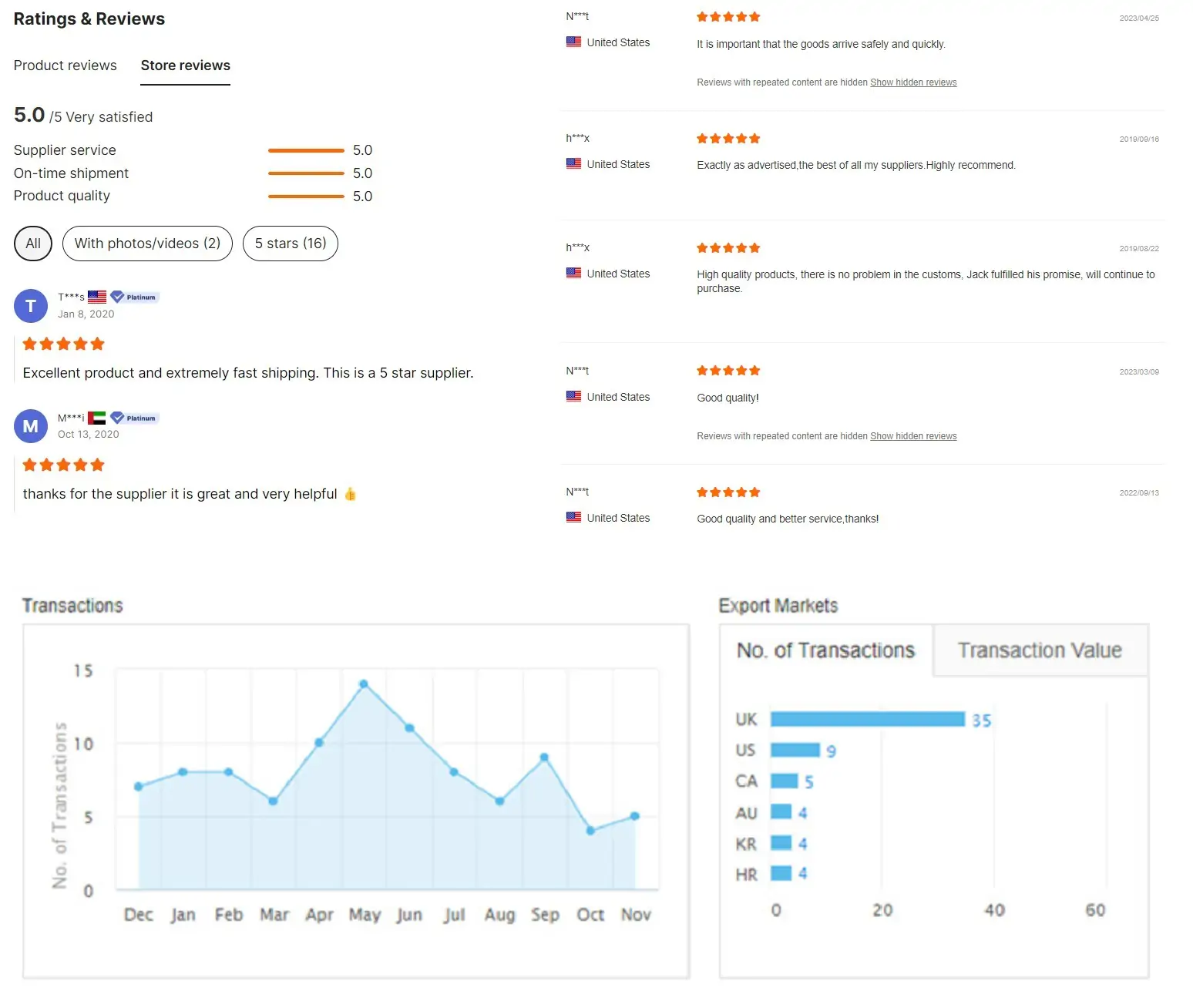
Customer feedback
Xi'an Yihui certificates

Xi'an Yihui Factory & Warehouse

Contact us
as a professional Baricitinib API manufacturer, we have advanced technology, scale advantage, the best quality, rich production experience, and excellent services. These advantages will make it stand out in the market competition and win more customer trust and support. if you need it, pls feel free to contact us any time. we will reply you asap.
our contact information:
E-mail: sales@yihuipharm.com
Tel: 0086-29-89695240
WeChat or WhatsApp: 0086-17792415937
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.











