Velpatasvir API
Appearance: White to slightly yellow solid
Standard: in-house
Application: Anti-Hepatitis C
Supply Ability: 100kg per month
Purity: ≥99%
Payment: T/T, LC or DA
Delivery Time: Ready Stock in Local Warehouse, 1-3 days
Origin: China
Shipping: DHL, FedEx, TNT, EMS, By Sea, By Air
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
We supply Velpatasvir API
We offer Velpatasvir API, which is a highly effective antiviral drug used in the treatment of hepatitis C. As a leading supplier, we guarantee high-quality and high-purity products that meet international industry standards. it is available in various quantities to meet your specific needs. With a strong focus on customer satisfaction, we ensure fast and efficient delivery to any location worldwide. Our experienced and dedicated team is committed to providing personalized service and support to ensure your success. Choose us as your trusted partner for Velpatasvir supply and experience the difference in quality and reliability.
What's Velpatasvir?
It is a novel pan genotype hepatitis C drug developed by Gilead Scientific. It is a composite tablet composed of NS5B inhibitor sofibuvir and NS5A inhibitor velpatasvir (VEL), taken orally once a day. In June and July 2016, the US FDA and the European Union respectively approved Epclusa for the treatment of adult patients infected with gene 1-6 hepatitis C virus. It can be used alone in patients without cirrhosis or compensatory cirrhosis, or in combination with ribavirin for the treatment of patients with decompensated cirrhosis. Vepatavir has achieved good therapeutic effects in patients with various genotypes, and is expected to exempt genotype testing and improve the cure rate of hepatitis C patients.
Basic Information
Product Name: Velpatasvir
CAS: 1377049-84-7
MF:C49H54N8O8
MW:883
EINECS:1592732-453-0
MDL No.:MFCD28411371
Structural formula:
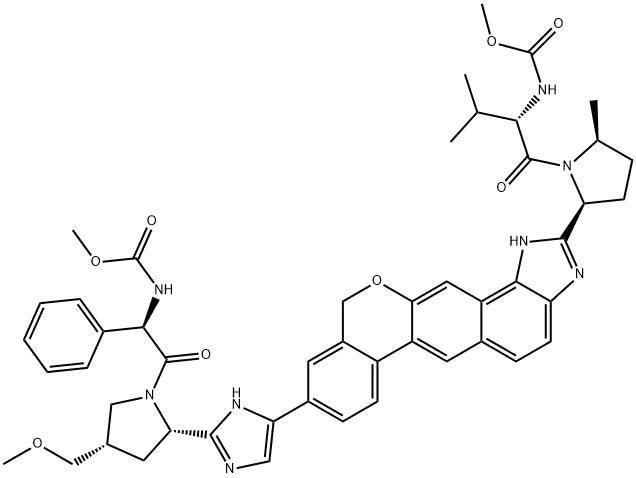
Origin: China
Application: Anti-Hepatitis C
Delivery Time: in stock
Technical Specification
|
Test Item |
Specification |
Results |
|
Appearance |
White to slightly yellow solid |
Off white solid |
|
Identification |
IR:Similar to reference substance B.Retention Time:Similar to reference |
Conform |
|
Water (Volumetric Karl Fisher) |
≤5.0%(w/w) |
1.03% |
|
Residue on ignition |
≤0.2% |
0.16% |
|
Heavy metals |
≤20ppm |
Conform |
|
Residual Solvents |
Dichloromethane≤600ppm |
Not detected |
|
Ethyl acetate ≤5000ppm |
Not detected |
|
|
Methanol≤3000ppm |
790ppm |
|
|
Heptane ≤5000ppm |
100ppm |
|
|
Related substances by HPLC |
HPLC purity≥99.0%(A) |
99.47% |
|
Any individual impurity≤0.3%(A) |
0.16% |
|
|
Assay on anhydrous basis
|
98.0~102.0%
|
99.14% |
|
Solubility |
Soluble in Methanol and Acetonitrile, Slightly soluble in Isopropyl Alcohol and 0.1N Hydrochlric acid, insoluble in water |
Conform
|
|
Melting point |
170℃-190℃ |
179℃
|
|
Bulk density |
/ |
0.22 g/mL
|
|
Conclusion:Complied with the specification |
||
Package: 100g;1kg; or according to your demands
Storage conditions: 2-8 ℃, dark, moisture-proof, sealed and dry
Transportation conditions: Transportation at room temperature
Shelf Life: 24 months
Biological activity
Velpatasvir API is a largely potent and picky asset of the hepatitis C contagion( HCV) proteins NS5A. It belongs to a class of medicines known as direct- acting antivirals( DAAs) and is used in combination with other HCV medicines to treat habitual HCV infection in grown-ups. In clinical trials, c has shown to achieve high rates of sustained virologic response( SVR), which means the contagion is no longer sensible in the bloodstream after treatment.
its unique medium of action means it can be used to treat all six major HCV genotypes, which is a significant advantage over aged curatives that were specific to certain genotypes. It is administered orally formerly a day and has a long tube half- life, allowing for formerly- diurnal dosing convenience.
it has also been set up to have a high hedge to resistance, which means it's less likely to develop medicine resistance over time. This is a pivotal point for cases who have preliminarily failed treatment with other HCV medicines.
It has been considerably studied in clinical trials and has been approved by nonsupervisory authorities worldwide as an essential element of HCV treatment. It's frequently used in combination with other DAAs, similar as sofosbuvir or glecaprevir/ pibrentasvir. It has proven to be safe and well- permitted, with many side goods reported.
In summary, it is a largely effective and safe DAA that, when used in combination with other HCV medicines, can cure HCV infection in a wide range of cases. Its unique medium of action, high hedge to resistance, and accessible formerly- diurnal dosing make it a favored treatment option for HCV infection.
Application
Velpatasvir is a largely potent active pharmaceutical component that's extensively used in the expression of several life- saving specifics. This API is a new NS5A asset that acts as apan-genotypic agent for the treatment of habitual hepatitis C contagion( HCV) infection in grown-ups. It's largely effective in curing HCV, especially when administered in combination with other antiviral specifics.
it is a nonstructural protein 5A( NS5A) asset that inhibits the replication of HCV in the host's liver cells. It works by binding to and altering the NS5A protein, which is an essential element in HCV replication, therefore inhibiting the spread of the contagion. This API has demonstrated high efficacity in all six genotypes of HCV, which makes it a potent armament in the fight against this contagion.
Its use in medicine phrasings has been proven to ameliorate patient issues significantly. This API is generally specified along with other antivirals similar as Sofosbuvir and Daclatasvir, performing in high cure rates. Its unique medium of action andpan-genotypic exertion have made it a favored choice for HCV treatment.
it is manufactured to meet high- quality norms and is available in colorful forms similar as tablets and capsules. It's a potent API, and a low cure is sufficient to achieve high treatment efficacity. The API's stability and bioavailability make it ideal for use in colorful medicine phrasings.
In conclusion, it is an essential and largely effective element in treating HCV. Its unique medium of action andpan-genotypic exertion make it an necessary tool in the fight against this contagion. Its efficacity, bioavailability, and quality norms make it suitable for use in colorful medicine phrasings.
Why Choose Xi'an Yihui?
Customer feedback
Xi'an Yihui certificates

Welcome to Xi'an Yihui Factory

Our Advantage
Rich experience: we have 13 Years of professional experience;
Customers all over the world: sell to more than 100 countries;
Provide diversified products: the products have been applied to all major international brands in the fields of drugs, dietary supplements, cosmetics, animal nutrition and functional food.
Price advance: low MOQ with competitive price;
Quality certification: ISO; Halal; Kosher certified
After-sales service: Professional team 7*24 hours customer service.
Contact Us:
Yihui takes pride in being a trusted Velpatasvir API manufacturer and supplier. To acquire this groundbreaking product or discuss your specific requirements, contact us immediately at sales@yihuipharm.com. Rest assured, with Yihui, you're accessing a product of unparalleled quality, backed by certifications that underscore our commitment to excellence in the global market.
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.










