Gadobutrol API
Appearance: White or off-white powder
Standard: USP
Supply Ability:1000kg per month
Shelf Life: Two years
Payment: T/T, LC or DA
Delivery Time: Ready Stock in Local Warehouse, 1-3 days
Origin: China
Shipping: DHL, FedEx, TNT, EMS, By Sea, By Air
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
We supply Gadobutrol API
We're proud to offer Gadobutrol API, a high- quality product that's designed to ameliorate medical imaging. Our product is strictly tested to insure that it meets strict quality norms, making it a dependable choice for medical professionals. With its fast- amusement, effective results, it ideal for a wide range of imaging applications. Whether you're working in radiology, cardiology, or oncology, our Gadobutrol is the perfect choice for your imaging needs. Trust us to give you with the stylish quality API that delivers harmonious, dependable results every time.
What's Gadobutrol?
Gadobutrol containing discrepancy agent gadolinium butoxide is used for nuclear spin tomography imaging. Since 2000, it has been approved in Germany as a Gadovist for suggestions of discrepancy improvement in cranial and spinal glamorous resonance tomography( MRT). Gadolinium butoxide is anon-ionic complex composed of gadolinium( III) and macrocyclic ligand 10-( 2,3- dihydroxy- 1-( hydroxymethyl) propyl) acid( butrol). Gadolinium containing discrepancy agents have come the most generally used MRI discrepancy agent in clinical practice, with gadolinium grounded discrepancy agents being the sixth approved by the US Food and Drug Administration( FDA) for central nervous system resonance imaging.
Basic Information
Product Name: Gadobutrol
CAS: 138071-82-6
MF:C18H31N4O9.Gd
MW:604.712
EINECS:1308068-626-2
MDL No.:MFCD00911649
Structural formula:

Origin: China
Application: contrast agent
Delivery Time: in stock
Technical Specification
|
Items |
Standards |
Results |
|
Appearance |
White or off-white powder |
Off-white powder |
|
Identification |
By HPLC |
Complies |
|
Melting Point |
120ºC~130ºC |
126ºC |
|
Loss on Drying |
≤1.00% |
0.21% |
|
Heavy Metals |
≤ 10ppm |
Complies |
|
Sulfated Ash |
≤ 0.10% |
0.03% |
|
Chlorine Content |
17.5%~19.5% |
19.3% |
|
Arsenic Content |
≤ 0.1ppm |
0.09ppm |
|
Lead VContent |
≤ 3.0ppm |
0.50ppm |
|
Cadmium Content |
≤ 0.1ppm |
0.10ppm |
|
Content |
≤ 0.1ppm |
0.10ppm |
|
Microbiological Test |
Coliforms:Negative |
Negative |
|
Salmonella: Negative |
Complies |
|
|
Total plate count: ≤1000 CFU/g |
Pass |
|
|
Yeast&Mold: ≤50 CFU/g |
Pass |
|
|
Related Substances |
Impurity A: ≤0.30% |
0.18% |
|
Other unknown impurity: ≤0.10% |
0.07% |
|
|
Total Impurities: ≤0.50% |
0.42% |
|
|
Assay |
≥ 98.0% |
99.50% |
|
Reference Standard |
USP Standard |
|
|
Conclusion |
The product complied to USP standard. |
|
Package: 1kg; 5kg;25kg
Storage: Preserve in tight,light-resistant containers in a cool place
Shelf Life 24 months
Application
Gadobutrol API is a gadolinium- grounded discrepancy agent( GBCA) used in glamorous resonance imaging( MRI) procedures to enhance the visibility of blood vessels and apkins. It's one of the newer and more advanced GBCAs available, offering several advantages over aged agents. Then are some crucial applications :
individual Imaging: it is primarily used in individual imaging to ameliorate the clarity and delicacy of MRI reviews. By enhancing the discrepancy between different apkins and organs, It helps radiologists and healthcare providers to descry and diagnose colorful medical conditions more effectively. It's particularly useful in imaging lesions, excrescences, and abnormalities in the brain, chine, liver, feathers, and other organs.
Brain Imaging In neuroimaging: it is constantly employed to assess brain excrescences, vascular abnormalities, multiple sclerosis( MS) lesions, and other neurological conditions. Its high signal intensity and dragged improvement characteristics make it well- suited for imaging the intricate structures of the brain and spinal cord.
Body Imaging: it is also employed in imaging studies of the body, including the tummy and musculoskeletal system. It helps to punctuate blood vessels, organs, and soft apkins, easing the opinion and evaluation of conditions similar as liver metastases, pancreatic excrescences, renal lesions, and musculoskeletal injuries.
Cardiac Imaging In cardiac MRI: it enhances the visualization of cardiac structures and blood inflow, abetting in the assessment of myocardial viability, myocardial perfusion, and natural heart conditions. It enables clinicians to gain detailed images of the heart and identify abnormalities in its structure and function.
Pediatric Imaging: it is considered safe for use in pediatric cases, making it precious for imaging studies in children and adolescents. It helps healthcare providers gain high- quality images while minimizing the pitfalls associated with discrepancy administration, similar as antipathetic responses and nephrogenic systemic fibrosis( NSF).
Dynamic Differ- Enhanced MRI( DCE- MRI): it is frequently employed in DCE- MRI studies, where successional MRI reviews are performed ahead, during, and after the injection of discrepancy agent to assess towel perfusion, vascular permeability, and excrescence response to treatment. This fashion is particularly useful in oncology for assessing excrescence characteristics and monitoring treatment efficacity.
Overall, gadobutrol plays a vital part in ultramodern individual imaging, offering superior discrepancy improvement, excellent safety profile, and versatility across colorful clinical applications. Its wide use has significantly contributed to the advancement of medical imaging and the opinion of multitudinous medical conditions.
Preparation
Take5.0 g of Ringed Tengning( I) and1.85 g of lithium chloride into a three necked bottle, add 30 ml of isopropanol, toast up to 80- 85 ℃, influx for 24 hours, and concentrate under reduced pressure until dry. Add 30 ml of anhydrous THF to the concentrated result, stir and dissolve. Add5.0 g of 4,4- dimethyl(5.1.0) octane( II), toast up and influx for 5 hours, reduce pressure to dry, add methyl tert butyl ether for influx crystallization, sludge and vacuum sot at 50 ℃ to gain8.1 g of intermediate( IV). Add8.0 g of intermediate( IV) to 8 ml of purified water and stir to dissolve. Take another7.37 g of chloroacetic acid and dissolve it in 8 ml of purified water. Add3.27 g of lithium hydroxide monohydrate at-5 ° C, stir and dissolve. Add the lithium chloroacetic acid result dropwise to the intermediate( IV) result at room temperature, and raise the temperature to 65 °C. Add about2.8 g of lithium hydroxide monohydrate in batches within 3 hours. Control the pH of the response system to be lesser than 9 and continue the response for 2 hours. The response is completed and cooled to room temperature. Acclimate the pH to3.5 with hydrochloric acid. After stirring for0.5 hours, add 160 ml of ethanol, raise the temperature to influx, stir and solidify for 5 hours. Lower the temperature to room temperature, sludge, and vacuum sot to gain8.3 g of intermediate( IV). Add8.2 g of intermediate( IV) to 8 ml of purified water, stir and dissolve, acclimate the pH to3.5, add3.02 g of gadolinium oxide, raise the temperature to 95 ℃, reply for 4 hours, cool to room temperature, acclimate to neutral with lithium hydroxide, add 80 ml of ethanol dropwise, raise the temperature and influx, stir for crystallization for 5 hours, lower the temperature to room temperature, sludge, and vacuum sot to gain intermediate( VI), which is8.5 g of crude gadolinium alcohol. Add8.0 g of crude product to 8 ml of purified water, raise the temperature to 70 ℃, stir and dissolve, add 80 ml of ethanol dropwise, and complete within 1 hour. After adding the admixture, raise the temperature to influx, stir for crystallization for 2 hours to cool to room temperature, sludge, and vacuum sot to gain7.1 g of gadolinium alcohol. The total yield is 40.4%, and the HPLC chastity is99.9%.

Biological activity
Gadobutrol (Gadovist, Gadavist) is a non-ionic macrocyclic gadolinium based contrast agent (GBCA, gadolinium contrast agent) used for tissue contrast enhancement in magnetic resonance imaging (MRI).
Related news
People's Daily Online: There are no reports of adverse reactions to gadolinium deposition in the brain yet
In the database of the National Center for Adverse Drug Reaction Monitoring, the main adverse reactions caused by gadolinium containing contrast agents are urticaria, vomiting, allergic shock, etc., but there are no adverse reactions related to gadolinium deposition in the brain. Therefore, there is insufficient evidence to suggest that gadolinium bromide contrast agents can lead to adverse reactions of gadolinium deposition in the brain.
National Center for Adverse Drug Reaction Monitoring: Macrocyclic contrast agents can continue to be used in clinical practice
Although there is no evidence to suggest that gadolinium deposition in the brain can cause harm to patients, the European Medicines Agency still recommends suspending the marketing of three linear intravenous formulations, GBCA (gadolinium containing contrast agents), while gadolinium butoxide, as a macrocyclic (but non-linear) gadolinium containing contrast agent, can continue to be used in clinical practice.
Packing & Shipping
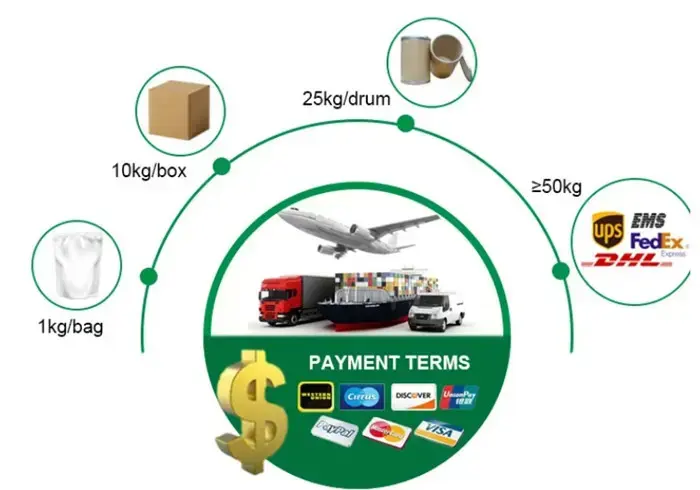
Packing:
1kg/foil bag;5kg/carton;25kg/fiber drum; or packing as your request.
Customization:
l Customized logo
l Customized packaging
l Graphic customization
Shipping:
By Courier; By Air or By Sea, according to your demands
Payment Term

Why Choose Xi'an Yihui?
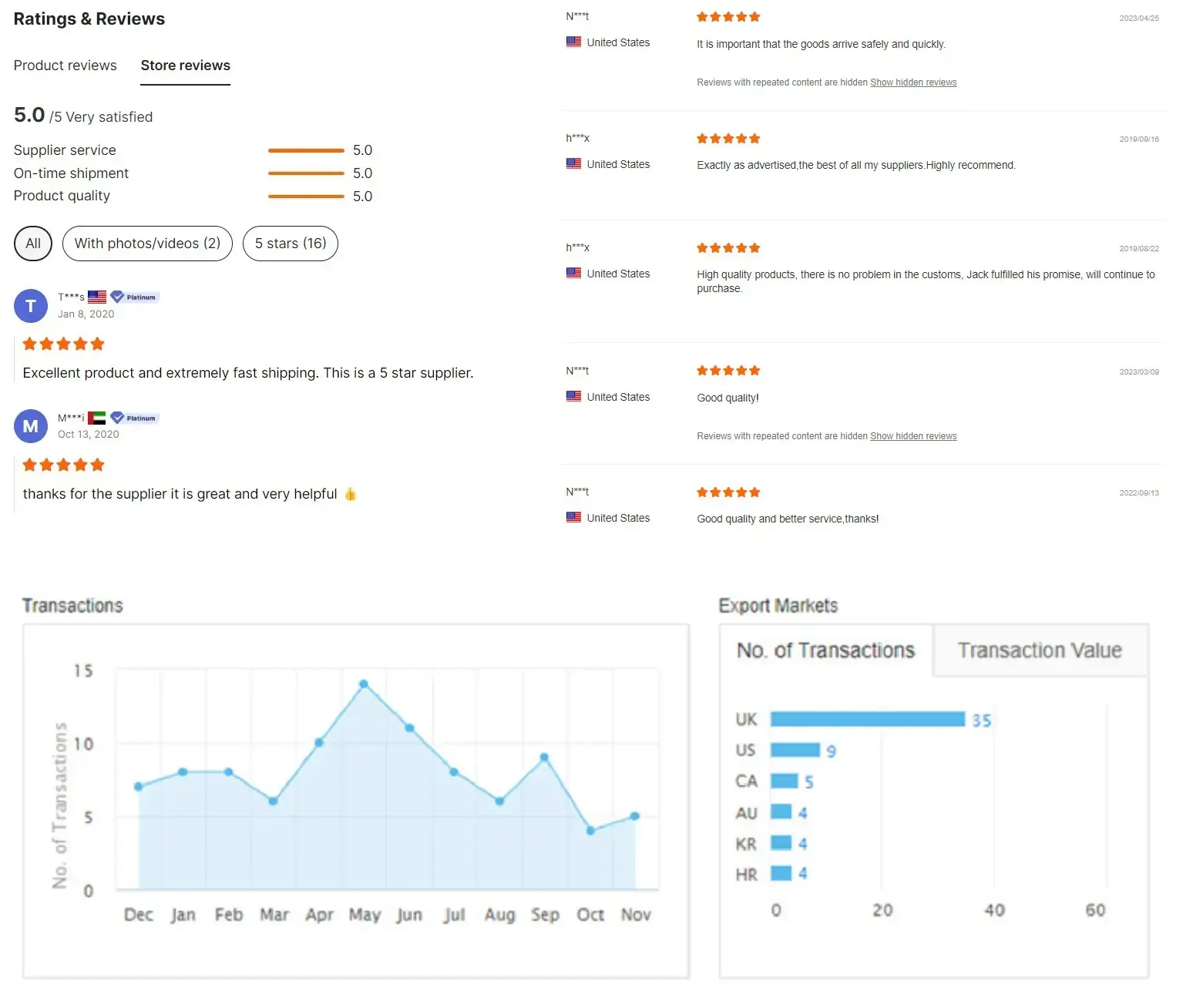
Customer feedback
Xi'an Yihui certificates

Welcome to Xi'an Yihui Factory

Our Advantage
Rich experience: we have 13 Years of professional experience;
Customers all over the world: sell to more than 100 countries;
Provide diversified products: the products have been applied to all major international brands in the fields of drugs, dietary supplements, cosmetics, animal nutrition and functional food.
Price advance: low MOQ with competitive price;
Quality certification: ISO; Halal; Kosher certified
After-sales service: Professional team 7*24 hours customer service.
Contact Us:
Yihui emerges as a beacon of trust in the pharmaceutical industry, consistently delivering high-quality Gadobutrol API that aligns with international standards. Our ISO, Kosher, Halal, and GMP certifications underscore our commitment to excellence. For inquiries and purchases, please contact us at sales@yihuipharm.com.
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.











