Azithromycin API
Appearance: White to off-white powder
Standard: usp/ep/bp
Application: Antibiotics Macrolide
Brand: YIHUI
Packing: 25kg
Supply Ability:10000kg per month
Shelf Life: Two years
Payment: T/T, LC or DA
Sample: available
Delivery Time: Ready Stock in Local Warehouse,1-3 days
Prompt and Secure Shipment
Origin: China
Shipping: DHL,FedEx,TNT,EMS,By Sea,By Air
Certifications: ISO9001, ISO22000, HACCP, HALAL, KOSHER
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
What is Azithromycin?
Azithromycin API is an antibiotic medication used to treat a wide range of bacterial infections. It belongs to the class of drugs known as macrolide antibiotics, which work by inhibiting the growth and reproduction of bacteria.
Basic Information
Product Name: Azithromycin
CAS: 83905-01-5
MF:C38H72N2O12
MW:748.98
EINECS:617-500-5
MDL No.:MFCD00873574
Purity: 99.99%
Package: 1KG;25KG
Used: research and pharma grade
Stability: Stable
Structural formula:
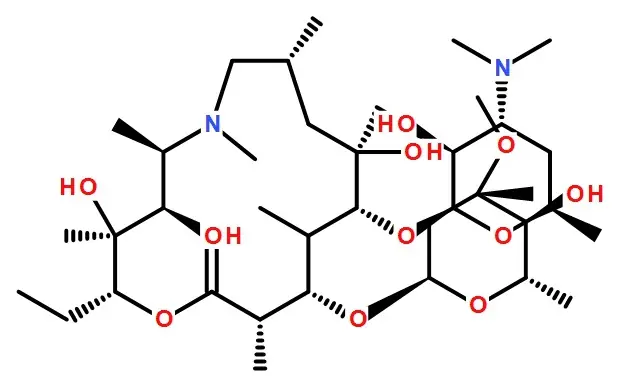
Specifications
Items | Specifications | Results | |
Appearance | White or almost white powder | Meets | |
Solubility | Practically insoluble in water,freely soluble in anhydrous ethanol and in methylene chloride | Meets | |
Identification | IR | According to standard | Meets |
HPLC | According to standard | Meets | |
Specific rotation | Between -45° and -49° | -46° | |
Crystallinity | Meets the requirements. | Conforms | |
pH | Between 9.0 and 11.0 | 9.7 | |
Related substances | Procedure 2 | ||
Impurity L,M | N M T 0.5% | ND | |
Impurity E,F | N M T0.5% | ND | |
Impurity J | N M T 0.3% | ND | |
Impurity I | N M T 0.7% | ND | |
Impurity C,H | N M T 0.5% | ND | |
Impurity N,A | N M T 0.5% | ND | |
Impurity P | N M T 0.2% | ND | |
Impurity O | N M T 0.5% | ND | |
Impurity G | N M T 0.5% | ND | |
Impurity B | N M T 1.0% | 0.1% | |
Any other Impurity | N M T 0.2% | 0.18% | |
Total Impurity | N M T 3.0% | 0.2% | |
Water | Between1.8% and 6.5%(Dihydrate:4.0%~5.0%) | 4.4% | |
Residue on ignition | N M T 0.3% | 0.02% | |
Heavy metal | N M T 0.0025% | <0.0025% | |
Residual solvent | Acetone: N M T 0.5%; Dichloromethane: N M T 0.06% | 0.2% 0.01% | |
Assay(HPLC) | Between 945ug/mg and 1030ug/mg(on dry basis) | 993ug/mg | |
Conclusion: This product conforms to requirements of USP42 | |||
Chemical Composition:
The chemical composition of it is meticulously crafted to meet pharmaceutical standards, ensuring both safety and efficacy. The key components include C38H72N2O12, contributing to its distinctive properties.
Effects and Functions:
Azithromycin as dihydrate plays a pivotal role in combating bacterial infections by inhibiting protein synthesis in susceptible organisms. Its broad-spectrum activity makes it effective against various bacteria, including respiratory and skin infections. This product is particularly renowned for its ability to accumulate in infected tissues, leading to enhanced therapeutic efficacy.
The product is usually utilized in the drug business for the union of anti-infection agents that treat respiratory lot contaminations, skin and delicate tissue diseases, and physically sent illnesses.Its effectiveness against Gram-positive and Gram-negative bacteria has positioned it as a cornerstone in the pharmaceutical arsenal against infectious diseases.
Synthesis Process:
A complex synthesis process is used by Yihui Pharmaceuticals to guarantee the quality and purity of Azithromycin powder. Beginning with painstakingly chosen unrefined substances, the blend includes a progression of exact synthetic responses and filtration steps. The cycle is intended to stick to rigid quality control measures, bringing about an end result of uncommon immaculateness.
Quality Standards:
Yihui is focused on conveying the result of the greatest quality. Our creation processes line up with worldwide norms, and we hold affirmations for ISO, Fit, Halal, and GMP. These certificates highlight our commitment to quality confirmation and administrative consistence, giving certainty to our clients in the drug business.Here are a portion of the quality principles that the product ought to stick to:
Purity: The product ought to have an elevated degree of immaculateness, ordinarily above close to 100%. Debasements or pollutants can influence the medication's adequacy and wellbeing.
Identification: It ought to be precisely recognized through different insightful strategies, like chromatography, spectroscopy, or mass spectrometry. This guarantees that the product and not another compound are the active ingredients.
A physical description: The product ought to have explicit actual properties, including appearance, variety, scent, and solvency. These qualities help in the ID and separation of the substance.
Microbiological quality: The product ought to be liberated from any microbial pollution, like microorganisms, parasites, or endotoxins. Severe adherence to microbiological quality principles limits the gamble of diseases or unfriendly responses in patients.
Solvents that remain: The International Conference on Harmonisation (ICH) guidelines, among other established limits, should govern the presence of residual solvents utilized during the manufacturing process. These cutoff points guarantee the wellbeing of the medication for human utilization.
Stability: Azithromycin API ought to keep up with its security and power over its time span of usability. In order to evaluate the drug's degradation and determine the most suitable storage conditions, stability testing is carried out under a variety of conditions.
Great Assembling Practice (GMP): The assembling system of the product ought to observe GMP rules, which guarantee that the product is reliably created and controlled by quality principles. GMP covers different angles, including office plan, gear alignment, faculty preparing, documentation, and quality control.

Application Fields in Various Industries:
83905-01-5 tracks down broad application in the drug business, where it fills in as a critical fixing in the plan of anti-infection agents. Its viability against a wide range of microbes makes it reasonable for treating different contaminations. Yihui's product is basic in the development of meds used to address respiratory lot contaminations, skin sicknesses, and physically communicated illnesses.
Packing & Shipping
Packing:
1kg/foil bag;5kg/carton;25kg/fiber drum; or packing as your request.
Customization:
l Customized logo
l Customized packaging
l Graphic customization
Shipping:
By Courier; By Air or By Sea, according to your demands
Payment Term

Why Choose Xi'an Yihui?
Customer feedback
Xi'an Yihui certificates

Welcome to Xi'an Yihui Factory

Our Advantage
Rich experience: we have 13 Years of professional experience;
Customers all over the world: sell to more than 100 countries;
Provide diversified products: the products have been applied to all major international brands in the fields of drugs, dietary supplements, cosmetics, animal nutrition and functional food.
Price advance: low MOQ with competitive price;
Quality certification: ISO; Halal; Kosher certified
After-sales service: Professional team 7*24 hours customer service.
Contact Us:
Yihui Drugs remains as a dependable and proficient Azithromycin granules maker and provider. Focused on greatness, our adherence to global norms and confirmations (ISO, Legitimate, Halal, and GMP) guarantees the conveyance of first rate items. For inquiries and purchases, please contact us at sales@yihuipharm.com.
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.
You may like






